About Us
Our charity aims to make a difference in its own small way.

Help Fund Research
Funding research and the development of kinder, targeted therapies

Offer Financial Support
Providing financial support to UK families of the newly diagnosed

Give Drew’s Gift
A present for the bedside of newly diagnosed children in the UK

About the charity
In Memory of Drew
The Drew Barker-Wright Charity was founded in 2018 in memory of 4-year-old Drew. Drew passed away in 2017 after being diagnosed with chordoma, a rare childhood cancer.
Run on a voluntary basis, The Drew Barker-Wright Charity is rooted in Drew’s gentle spirit, bravery and the belief that children with chordoma and other rare childhood cancers deserve a better pathway of treatment.
Childhood and cancer are two words that no parent wants to hear. But, unfortunately the rate of cancer in children is increasing and the cause of rare childhood cancers, like chordoma, are still unknown.
Our charity advisor
Eric Low OBE
We were put in touch with Eric when we considered setting up a charity as Drew’s legacy. Eric has worked for and with patient organisations for nearly 30 years and set up a well-respected cancer-related charity, Myeloma UK, in 1996, leading the organisation as Chief Executive until 2017. Eric is committed and focused on improving patient outcomes, putting patients at the centre of everything he does.
He advises numerous medical and health-focused charities, and following the establishment of Drew’s charity, we have been lucky to have him represent us as an ambassador and guide us through the development of the charity. He has helped develop the information we offer to patients and their families, as well as suggesting ways in which we can engage with various stakeholders involved in paediatric chordoma to collectively strive to improve the treatment and care of patients as well as the research conducted to find new treatments for this ultra-rare disease.
Eric talked us through our motives, the emotional pros and cons and the process of establishing Drew’s charity. He helped us navigate the complex legal process. He kept us on track when making important decisions about the aims and charitable purposes of the organisation and continues to guide us and provide valuable advice.
He has been our guardian angel, and we will always be indebted to him for his love, time and energy.

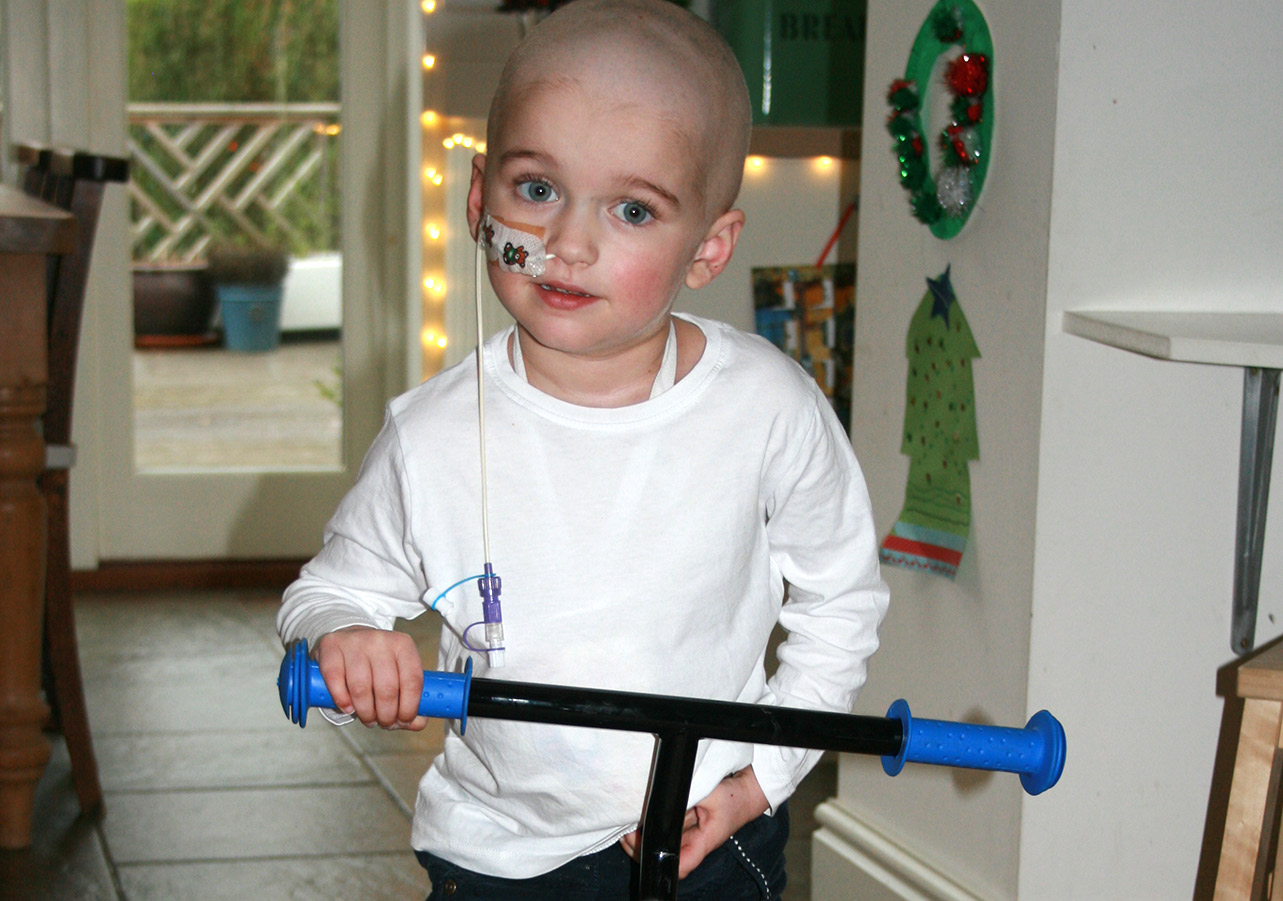
About Rare Childhood Cancer
Affecting 1 in 20 million children
Chordoma affects 1 in 20 million children annually. That’s approximately 3 children each year in the UK. Like other rare childhood cancers, there is a lack of clinical trials available and treatment options remain limited.
Cancer affects children differently to adults. This is because their bodies are still growing. Currently rare childhood cancers, including chordoma, are treated with the same drugs as adult cancers.
Over the past 20 years, the FDA has approved about 190 new cancer treatments for adults. Since 1980, fewer than 10 drugs have been developed for use in children and ONLY 3 have been FDA approved (FDA Report 2019).
Adult drugs can have unwanted side-effects on growing bodies because they affect normal cells as well as cancer cells. Long-term side-effects can include kidney, liver or heart problems, infertility and in some cases lead to secondary cancers.
The Drew Barker-Wright Charity, with your help, aims to promote rare tumour research and support development of new forms of treatment designed specifically for children, using a less invasive, targeted approach.
FUNDING RESEARCH
Helping progress Chordoma research
Only £3 in every £100 spent on cancer research goes to childhood cancers. Treatment options, cure rates and research for paediatric chordoma fall well beneath other childhood cancers.
We feel passionately about funding specific projects to help progress chordoma research and improve the chances of survival for other children and their families.
As chordoma is so rare it is important to identify all children with chordoma in the UK so that we can learn more about this disease. We hope this will represent the first step towards the initiation of clinical trials of promising new drugs for paediatric chordoma.

With your help we have raised and donated to The Chordoma Foundation & The Cancer Institute UCL
£120,000
PAST RESEARCH
Funding Specific Research Projects
To read more about the research that has been undertaken from our annual donations, please click on the relevant year below:
The Chordoma Foundation is one of main organisations continuing to push the boundaries concerning paediatric chordoma research. Consequently, the Drew Barker-Wright Charity felt it was important to continue with its financial support, and for 2021 have contributed £15,000 towards the ongoing projects currently taking place in the US.
Recent progress in research includes:
Model characterisation: In October, the CF’S newest paediatric chordoma mouse model, derived from a metastatic tumour, was validated. It is now available to research partners, and CF have begun to include it in their drug screening efforts. In addition, four new paediatric chordoma cell lines are now available for research; two of them were derived from successive tumour recurrences from the same patient, and thus provide a “progression model” that facilitates studies of chordoma evolution and disease progression. In addition, to optimise future drug screening studies, the CF recently initiated whole-exome DNA sequencing of their cell and mouse models derived from paediatric chordoma patients to better understand which genetic mutations drive each model. The results are expected to enable more sophisticated, molecularly-guided drug testing of therapeutic hypotheses that rely on the presence or absence of specific mutations.
Drug screening in paediatric models: This year the marked the launch of CF Labs: the first lab 100% dedicated to chordoma research. CF Labs is already enabling specialists to quickly pursue critical experiments, empowering their research partners to efficiently answer questions, and filling gaps that currently exist in the capabilities of academics and companies. CF Labs will serve to significantly speed research to translate insights about chordoma biology into new treatment options. The paediatric models that DBW Charity has helped the CF develop and characterise will ensure that paediatric chordoma patients stand to benefit. Several new concepts — drugs and combinations — have recently shown promise against chordoma tumours in paediatric mouse models. The CF is currently continuing to generate the preclinical data packages and foster company collaborations needed to move these concepts toward clinical trials.
This project is pushing boundaries in research, and so The Drew Barker- Wright Charity felt it was important to continue support forward into 2020. We have contributed £15,000 for 2020.
In 2019, with support from The Drew Barker-Wright Charity, the Chordoma Foundation analysed all available paediatric chordoma models for the presence or absence of three of the most important known molecular targets in chordoma; brachyury, p16 and INI1
Knowing the status of each of these proteins is important for understanding how representative the models are of the paediatric chordoma patient population, for guiding model selection for drug screening experiments, and for interpreting results of studies conducted in these models.
As a next step and moving forward for 2020, The Chordoma Foundation plan more in-depth model characterisation to evaluate the status of additional important targets in these models and to conduct genome-wide mutational and gene expression analysis to generate a more holistic picture of the molecular and genetic landscape of these models.
The Drug Screening Programme plans for two of the previously tested drugs to be tested in additional paediatric models. Furthermore, six more high priority drugs are in the queue for testing in paediatric models and they anticipate several more to be nominated by research collaborators this year. Every penny we contribute to this project allows quicker evaluation of these drugs, and shortens the timeline to determining whether any existing drugs may help patients with paediatric chordoma.
The Drew Barker-Wright Charity has been supporting the work of Adrienne Flanagan, her team at UCL, and the UK Chordoma Study for the last two years. For 2020 we have donated a further £15,000 to the project and to support the development of a blood test to monitor disease progression.
Dr Inga Usher, a clinical PhD student studying chordoma, has collected blood and tissue samples from paediatric and young patients as part of our National Institute of Health supported Chordoma Study. Funding from The Drew Barker-Wright Charity supported the sample collection and processing as described below.
1. Tumour samples from paediatric patients with chordoma
During the past 12 months the team have received 10 samples from children and young people with chordoma, 5 with matching DNA blood or saliva and 5 with only tumour tissue. DNA is being extracted from the 10 tumour samples and checked for quality using funding from Chordoma Foundation to which The Drew Barker-Wright Charity has also contributed.
2. Molecular analysis of samples from children and young adults
Wherever sufficient genomic material of adequate quality is available, the team will undertake various molecular analyses including genomic sequencing. They have also applied to access third party whole genome data from an additional 5 paediatric tumour samples that have already been sequenced. In total, they will have data for up to 18 (aged <5 – 23) patients to analyse. In the next 12 months the data will be analysed. Taken together, the data generated by these analyses will form the most comprehensive picture to date of chordoma in paediatric and young patients.
3. Blood tests to monitor disease
As part of Dr Usher’s PhD project, the team at UCL propose to develop a blood test to monitor progression of disease and predict clinical outcome for patients with chordoma. The aim would be to use this test to determine if a chordoma were fully removed at surgery and inform the doctors at an early stage if a tumour recurs.
The test could also potentially be used in drug trials for determining if patients with chordoma are responding to treatments. At present this can only be done by medical imaging such as CT and MRI, and these are not sensitive modalities for detecting small changes in response to therapies. Such tests have been developed for more common cancers and the team are developing a similar test for osteosarcoma, another bone cancer that occurs in children. They will use the experience that others have accrued to develop this test for chordoma.
They have already identified markers that can distinguish DNA from a chordoma and DNA from normal tissue. These markers can then be used to detect chordoma-derived DNA in blood samples. They have currently collected more than 120 samples from 40 patients (pre- and post-surgery).
The development of a blood test to help determine the progression of disease is something that The Drew Barker-Wright Charity feels passionate about. Currently young children have to undergo numerous MRI and CT scans to successfully track the progression of chordoma. Young children struggle to stay still for such scans, and so they have to be placed under general anaesthetic. During treatment, these scans can occur on a regular basis and continual use general anaesthetic can be a very stressful process for both child and parent. A move towards a blood test that could minimise MRI and CT scans will be invaluable to young chordoma patients in treatment worldwide.
1. Funding in-depth genomic molecular characterisation of two paediatric chordoma cell lines and PDX models. One confirmed INI1 deleted and one not deleted.
In 2017, thanks to the investment of a family affected by paediatric chordoma, the Chordoma Foundation (USA) was able to offer the research community a $25,000 prize for developing a paediatric chordoma cell line. The prize announcement was successful, and they now have a validated paediatric chordoma cell line available for distribution to researchers across the globe.
Additionally, they also have a corresponding patient-derived xenograft (PDX) model that was developed from the same initial tumor as the cell line. These resources are a valuable asset as they enable researchers to test the effects of drugs in the paediatric cell line and then advance promising drugs into the corresponding PDX model to look at the effects on tumor growth in the mouse model. To make these tools even more beneficial to the research community it will be important to perform in-depth genomic characterization of the two models. Performing this characterization could provide researchers with clues as to what drugs and combinations to test in the models and enable them to identify drugs that may work in patients with similar genomic alterations. Additionally, the genomic data for these paediatric chordoma models will be entered in a public data repository available to all researchers worldwide.
Therefore, researchers who are currently unaware of paediatric chordoma could become interested in studying the disease if they discover genetic alterations similar to ones they are studying in other cancers. We have approximately four additional paediatric chordoma cell lines that have been submitted for validation to confirm that they are truly chordoma.
2. Funding the testing of five drugs in paediatric chordoma PDX models
The purpose of developing validated and molecularly characterised paediatric chordoma cell lines and animal models is ultimately to identify new and/or better treatment options for children affected by paediatric chordoma. The Chordoma Foundation’s Drug Screening Program (DSP) enables researchers to quickly test drugs in animal models and determine if there is enough activity to warrant initiation of a chordoma-specific clinical trial or expansion of an existing trial in another cancer to include a chordoma-specific cohort. Given that we now have three validated paediatric chordoma PDX models – one confirmed to be INI1 deleted, one not deleted, and the third pending characterisation of INI1 loss they positioned to test drugs that have demonstrated promising results in adult chordoma cell lines to determine if they can affect tumor growth in the xenograft models. Additionally, if they reserve testing to drugs that are in clinical development or approved for other cancer indications, they can rapidly advance promising drugs that show activity in these preclinical models to the clinic so that the youngest members of the community can benefit from this research as soon as possible.
3. Continuing to fund the National UK Chordoma Cohort Study
A lot of progress has been made over the last year. In January 2018, Understanding Chordoma: A National Cohort Study opened at centers across England. More than 50 participants with a diagnosis of chordoma have joined the study since then. Participants are being followed for up to eight years after enrolment, during which time clinical data is collected alongside tissue samples and blood samples. These samples are/ will be genetically analysed to provide more information about what we know about what causes and drives chordoma, and why is it resistant to many therapies. The aim of the study is to understand the molecular basis of the disease so that we can develop new tests for early diagnosis, treatment and surveillance.
Since the study opened, over 25 tumor samples have been submitted for whole genome sequencing, RNA sequencing and methylation analysis. A manuscript has also submitted which goes some way to explain regulation of brachyury (TBXT) in chordoma; the study has also identified a ‘tool’ compound which appears to silence brachyury (TBXT) in the laboratory. Work is now needed with Pharmaceutical companies to see if such tool compounds can be transformed into therapies for the clinic – this is not an easy task – but progress has been made. This manuscript is: “Epigenetic inactivation of oncogenic brachyury (TBXT) by H3K27 histone demethylase controls chordoma cell survival” (bioRxiv 432005)
The National Cohort Study has initiated the formation of a UK network comprising of patients, their families and medical health care workers, through which it aims to deliver a better and a more standardised management of patients with chordoma, in both children and adults. The hope is that this chordoma network will be the legacy of the study, facilitating the sharing of expertise in this rare disease for years to come. At present, a Clinical Research Fellow, funded by Chordoma UK, travels to centers to meet teams and help to establish the network. The Chordoma Foundation along with The Drew Barker-Wright Charity supports the basic research associated with the National UK Cohort Chordoma Study. The Drew Barker-Wright Charity has a specific focus on Paediatric Chordoma.
The research program involves the recruitment of patients with chordoma of all ages. There is a focus on undertaking research on chordoma from paediatric patients. However, the rare occurrence of chordoma in children makes this challenging but, it is believed that understanding chordoma occurring in any age group would be beneficial to the chordoma patient and health care community at large.
This year will see more centers opening in the National Cohort Study, with further recruitment; working hard to strengthen and expand the clinical network with a focus on centers that see paediatric patients with chordoma. Finally, the project looks forward to analysing the results generated during the past twelve months and sharing these findings.
In January 2018 and The Drew Barker-Wright Charity made its first contribution, of £30,000, to paediatric chordoma research. below are some images of our recent visit to the lab to meet the team.
Finding the correct project to support was a difficult task but, with the help of The Chordoma Foundation, we were made aware of Professor Adrienne Flanagan and her chordoma project at the Cancer Institute, University College London.
The focus of her research with the funding that she receives is to study the genetic, epigenetic and transcriptomic changes found in the chordoma’s of children and adults.
As chordoma’s are extremely rare, particularly in children, one of the focuses of the project will be to compare the alterations in paediatric and adult chordomas. This will be achieved by obtaining samples from patients, which is taking place alongside the 100,000 Genomes Project run by NHS England. The aim is to prepare the data sets of these studies which will subsequently be analysed. Such detailed analysis has not previously been done on chordoma.
To make the research more sustainable Dr. Flanagan is setting up a UK-Wide Cohort of patients with chordoma, with the aim of following their outcome over 5-7 years and correlating outcome with genomic / epigenetic and transcriptomic data.
She is now collecting nearly all of the chordoma cases throughout the UK. This is a huge undertaking and also involves Chordoma UK, who are funding staff to biobank samples for the next year. They are also funding a clinical researcher to engage with patients over the country to undertake clinical research for one year. The US based Chordoma Foundation are also providing her with a number of paediatric samples from their Biobank. In the next year we should begin to get answers about the differences and similarities between adult and paediatric chordoma.
Another of the UCL project’s objectives is characterising children and adult chordoma at a molecular level and to understand the disease by testing the effect of a new family of drugs on established chordoma cells lines.
The recently announced launch of a new clinical trial proves that chordoma research is producing results. The Afitinib trial is partly the result of work done by Adrienne Flanagan. Supported by the US based Chordoma Foundation, the trial will soon open at University College London, in addition to Leiden and Milan. While these trials are currently for adult patients, better understanding the similarities and differences between adult and paediatric chordoma may help provide rationale for expanding these trials to include children.

Drew's Garden
A garden in Drew's Memory
Drew spent many happy hours at Talgarth Mill, drinking Babyccino’s and eating Bara Brith in the cafe over looking the river. Planting a small garden there, in his memory, seemed the perfect choice.
With the support of Talgarth Mill we were able to transform the front riverside garden into an oasis of loveliness for everyone in Talgarth to enjoy; and to help raise awareness of rare childhood cancer. From the very beginning it has been a haven for birds, bees and insects. We plan to continue to add to the garden over the coming years, with seasonal touches wherever possible.
Our thanks to Talgarth Mill, and to the Old Railway Line Garden Centre and The Walled Garden, Treberfydd for the supply of plants.
